Virulence and Drug Resistance of Burkholderia Species Isolated from International Space Station Potable Water Systems
Microbial surveillance of the potable water system (PWS) of the International Space Station (ISS) has been implemented by National Aeronautics and Space Administration (NASA) to ensure crew health within this unique closed environment. The PWS in combination with the potable water dispenser (PWD) is a water recycling system that utilizes physical and chemical techniques to filter, decontaminate, and sterilize water used for drinking and food hydration.
These surveillance efforts, which use standard culturing techniques, have been conducted over twelve years and 22 missions and began shortly after the PWD was launched in November 2008. On-orbit operations using the PWD began in early 2009 and continue to this day. The organisms Burkholderia cepacia and Burkholderia contaminans, both genomovars of the Burkholderia cepacia complex (BCC), have been frequently cultured from the PWD of the ISS.
Our project, as part of NASA’s Space biology program, under the Space Act Agreement with Alfred P. Sloan Foundations’ Microbes of the Built Environment initiative, focused on characterizing the organisms collected from the PWD.
Burkholderia
Burkholderia species are known to propagate in both nutrient-poor and -rich soil environments, in addition to within human host cells. Representatives of these species are considered opportunistic pathogens as they are commonly isolated from soil, plant rhizospheres, and water, as well as from the sputum of cystic fibrosis (CF) patients with chronic infection. BCC members pose a significant threat to individuals with CF (accounting for 85% of all infections in CF patients) and otherwise immune-compromised patients due to an exacerbation of pulmonary infections, which can lead in extreme cases to death.
The antibiotic resistance and intracellular survival capabilities of BCC members make them resistant to many therapeutics. Burkholderia spp. are known to withstand disinfection and sterilization procedures as they display a moderate to high-tolerance to stress such as UV-C radiation, antibiotics, and high heavy-metal concentrations and are known to survive long periods in distilled water. This ability to survive in distilled water with minimal additives has made them problematic for healthcare, as hospital-acquired BCC infections can arise from contaminated disinfectants, anesthetic solutions, distilled water, and aqueous chlorhexidine solutions.
The Study
The PWD unit of the ISS was assembled in a cleanroom facility and then primed on Earth using an extensive process to ensure no gas bubbles existed within the lines that could lock the apparatus upon installation in orbit. During build and delivery on planet, the system was maintained using a 20 to 30 ppm iodine and a 6% hydrogen peroxide solution flush. The primed system sat dormant for 6 months before installation on the ISS.
In spite of the effort to keep the equipment sterile, at some point it became contaminated. When microbial surveillance was conducted on the system after installation the bacterial load was 85 CFU/mL, which exceeded the 50 CFU/mL limits set for ISS potable water, leaving the sole source of water on the US module out-of-order.
The system was flushed with the biocide iodine (I2), first at what turned out to be a sub-inhibitory concentration of 4ppm, as subsequent measurements revealed an increase in the microbial load. Further testing revealed that 40ppm was the necessary concentration of iodine flush to achieve the drinkable 50 CFU/mL maximum bacterial load.
Iodine flushes are still intermittently administered to the system after durations of PWD stagnation. These flushes may have reduced the overall microbial load of the PWD while inadvertently selecting for the Burkholderia species within the system. In our work, we analyzed the genotypes and phenotypic responses of 19 B. cepacia and 5 B. contaminans isolates collected between January 6, 2010 during mission 22 and August 6, 2014 during mission 40.
We found that isolates for both species, B. cepacia and B. contaminans recovered from the ISS PWD had highly similar genomes regardless of isolation date. This suggests to us that each population likely stemmed from two distinct founding strains and is supported by the observation that all isolates demonstrated a greater than 99% ANI with 95-99.9% alignment in all cases, and share a core pangenome of greater than 90% of identified gene clusters (within species).
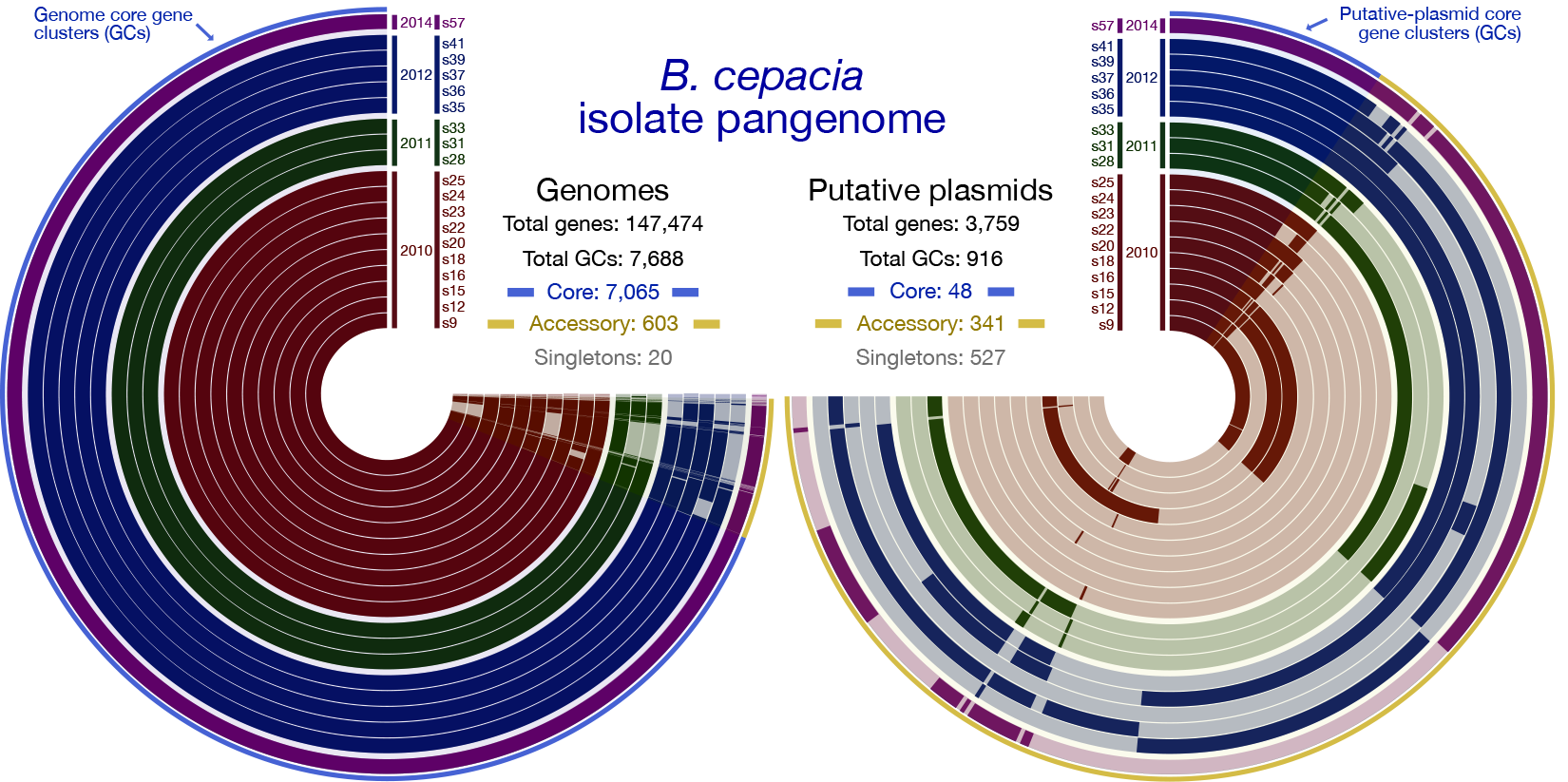
The vast majority of differences in gene-content that were identified in the pangenome were localized on putative plasmids (these are extrachromosomal elements that bacteria can exchange like playing cards to confer genes beneficial for survival) (Figure 1). The phenotypic profiles of the ISS-derived isolates which included their antibiotic sensitivities, ability to form biofilms, and ability to lyse or live inside the primary immune cell macrophage, were overall similar to each other, as well as to the terrestrial reference type-strains analyzed.
Conclusion
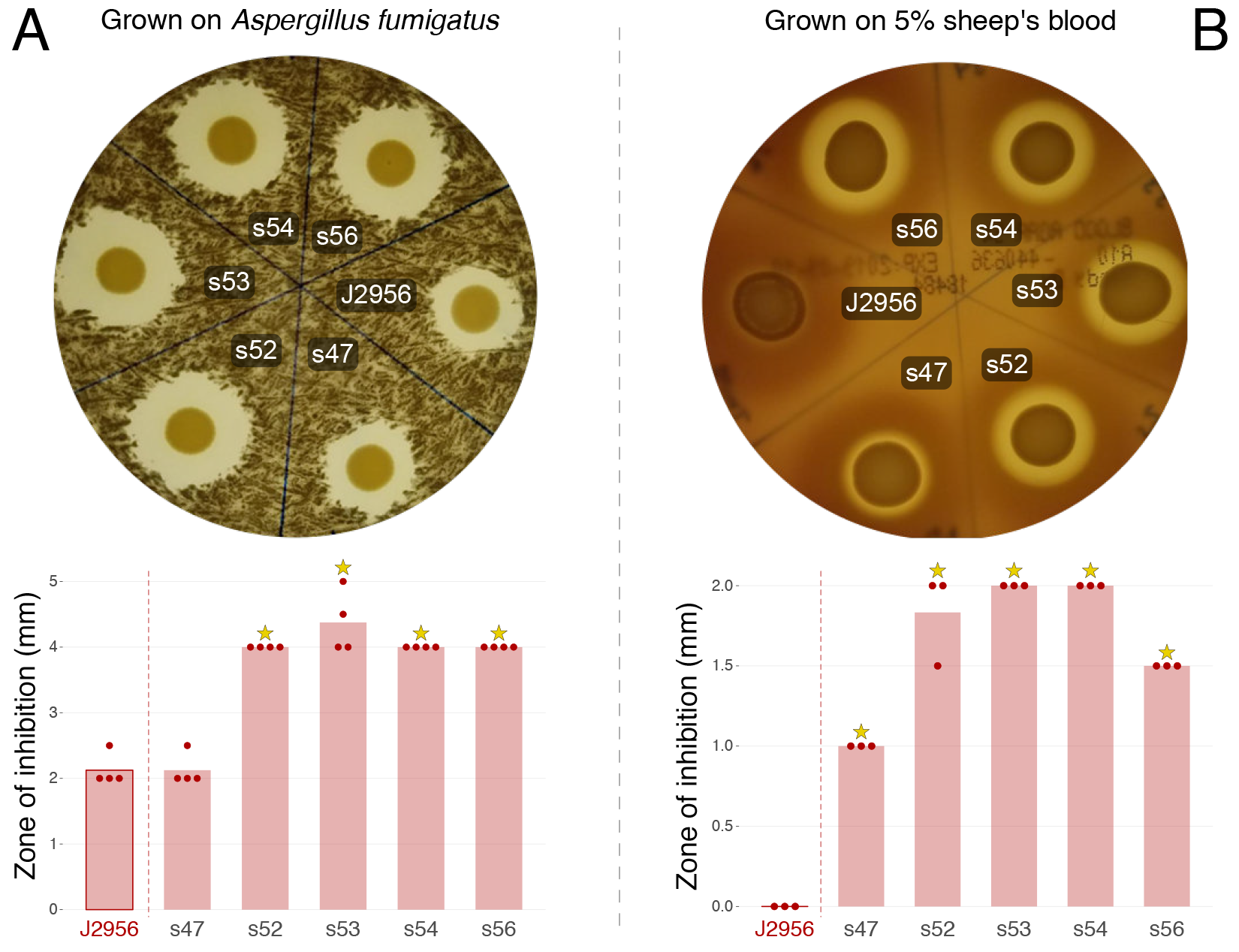
Based on our analysis, it seems likely that the two populations of Burkholderia present in the ISS PWS are not more virulent than those that might be encountered on planet. However, they do maintain a baseline of virulence. In addition, the ISS B. contaminans, display the ability to inhibit growth of the fungus Aspergillus fumigatus and demonstrate hemolytic properties (Figure 2) likely due to their ability to produce the antifungal metabolites, occidiofungin and pyrrolnitrin. This, in addition to their ability to lyse macrophage, suggests an overall cytotoxic capability.
This work, in the context of space life-support engineering, demonstrates that is not quite enough to say that the water is safe-for-human consumption because it harbors an arbitrary bacterial load deemed safe, but instead it also matters what type of bacteria comprises this load. We further showed that an alternative to iodine, silver (I) fluoride, that has been considered for future PWD microbial disinfection will likely also reduce this microbial load; however, we find that the ISS B. cepacia isolates analyzed in this study all harbored a Cu/Ag efflux pump on a conjugative plasmid. Such conjugative plasmids could be shared among PWD resident bacteria and provide the ability to pump silver out of an affected cell. Experimental designs testing the effects of the iodine disinfection process, and how silver and other treatments compare, would be of value moving forward. We envision that our roles in this would be to generate a transposon mutant library from an ISS isolate and subject the library to iodine and silver (I) fluoride treatments to investigate which genes may aid in survivorship.
The full results from the study can be found in PLOS ONE, Genomic and phenotypic characterization of Burkholderia isolates from the potable water system of the International Space Station.
Team
During a fellowship, Dr. Aubrie O’Rourke hosted two teams of University of California, San Diego, Senior Engineering Design teams and together worked with Associate Professor Chris Dupont and NASA Ames postdoctoral fellow, Michael Lee, to investigate this microbiological research question from an engineering perspective.
UCSD Senior Design Engineering Group 2017-2018 comprised of Kristine Khieu, Shirli Cohen, Victoria Thai, Sydney Anderson, and Henry Lee presented their work at the 2018 Evolutionary Biology meeting in San Diego.
UCSD Senior Design Engineering Group 2018-2019 comprised of Zhi Lee, Marissa Vecina and Kysha Mercader helped to author a chapter entitled, “Following the Astrobiology Roadmap: Origins, Habitability and Future Exploration” published in a book entitled, Astrobiology: Current, Evolving and Emerging Perspectives.