Diatoms Have Found a Way to Pirate Bacterial Iron Sources, JCVI Scientists use CRISPR Technology to Discover How
In large regions of the world’s oceans, photosynthesis struggles to operate because a key ingredient is missing. Many of the proteins involved in harvesting energy from sunlight require iron atoms to function, but iron is hard to find in seawater. Most of the ocean is far removed from sources of iron, and the chemistry of seawater prevents iron from dissolving and becoming bioavailable to microorganisms. Nevertheless, phytoplankton do persist in these regions and when iron does become available, they bloom in large numbers. One of the most successful groups of phytoplankton to do so are diatoms. These organisms frequently dominate the biomass of iron-fueled blooms in part due to their ability to tolerate low iron conditions and capitalize on iron when it is plentiful. This means that the flux of iron across the membrane of a diatom cell controls the amount of photosynthesis which can occur in many ocean ecosystems. Photosynthesis is the foundation of the food web and determines how much carbon is drawn from the atmosphere and sequestered into sediments. Therefore, the biochemical processes by which diatoms acquire iron have global biogeochemical consequences. This is the rationale behind studying the iron uptake capabilities of diatoms.
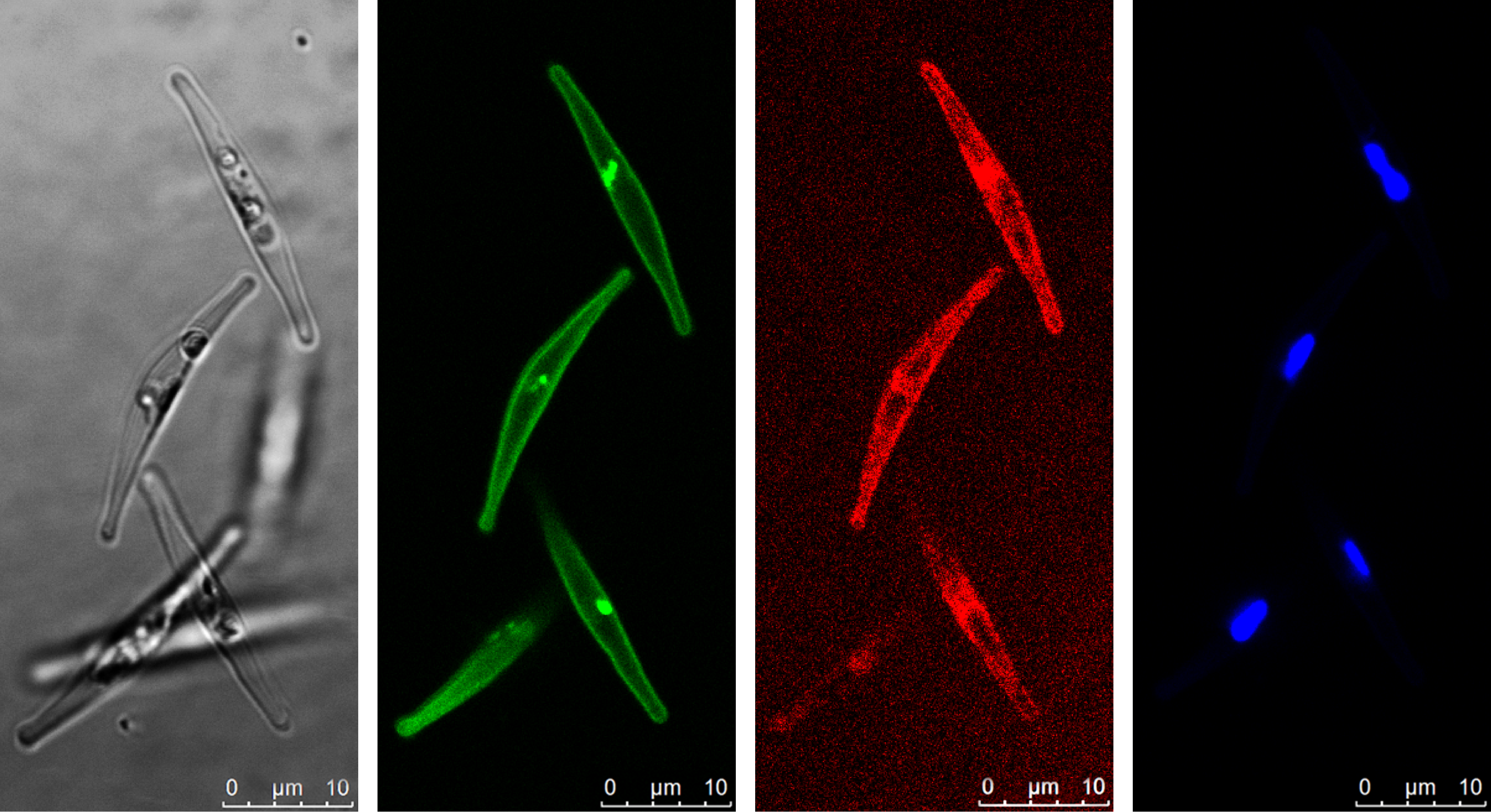
Diatoms, like all organisms, use specialized proteins to acquire the iron they need. The few sequenced diatom genomes contain many genes that resemble the iron acquisition genes of yeast, but very few have ever been studied in detail. A major breakthrough occurred in 2018 when a student in our lab published the discovery of phytotransferrin. This protein was discovered in diatoms and shown to effectively acquire iron from seawater at very low concentrations, provided there is also plenty of carbonate to aid in iron-binding. This potentially presents a problem to diatoms facing decreasing carbonate concentrations due to ocean acidification. Luckily, diatoms have another way to get their iron.
In our new paper, Reduction-dependent siderophore assimilation in a model pennate diatom, published in PNAS, we reveal details of an alternative to phytotransferrin iron uptake that relies on two different proteins. We characterized these proteins by deleting them using CRISPR/Cas9 and then tested how the diatoms used iron. We also fused fluorescent markers to these proteins and observed under the microscope where the proteins occurred. The first is a protein capable of binding siderophores in seawater. Siderophores are small compounds which can increase the concentration of bioavailable iron in seawater and help dissolve iron from minerals. These compounds facilitate iron uptake, usually for the organism that produces them. In this case, the diatom is extracting iron-containing siderophores from seawater but leaving the synthesis up to other microbes. The second protein involved comes from a protein family involved in iron redox chemistry. This means it can change the redox state of iron which determines how it interacts with other molecules. By changing the redox state of an iron atom contained in a siderophore, the iron can be coaxed out and used by the diatom. So, this uptake system works by binding siderophores with one protein and extracting the iron with another, no carbonate required.
Iron redox proteins are common in diatoms, and in other eukaryotic organisms. It wasn’t much of a surprise to find one performing this type of a role in a diatom. The siderophore binding protein, however, comes from an entirely different evolutionary history. The details are unknown, but somehow this gene migrated from a bacterial genome and found itself in that of a diatom. It was then coopted, combined with the redox protein, and formed this uptake system which is capable of supplying all the iron a diatom needs. The one catch is that siderophores need to be around to make the system work, and diatoms don’t produce them. Essentially, this uptake system is built around an interaction between organisms. If the diatom simply steals iron from siderophore producers, it might be considered a parasitic interaction. It could be the case that siderophore producers like bacteria benefit from supplying iron to phytoplankton and increasing the rate of photosynthesis. This could mean more food for bacteria, essentially trading iron for carbon, and the interaction would be considered mutualism.
The details of the iron economy among marine microbes are only beginning to be unraveled, and it remains to be seen how these proteins might mediate ecological interactions. For now, we can say that diatoms have multiple options to get the iron they need, and this likely contributes to their success in marine environments.